This week we announced that we have supported the BioPhorum Operations Group in the development of the first edition of The Technology Roadmap for the Biopharmaceutical Manufacturing Industry.
So why did we get involved?
As we all know, current trends in the biopharmaceutical industry, including continued market growth, the arrival of new product groups, cost pressure and the trend toward localized manufacture are exerting unprecedented pressure on biomanufacturers to innovate biomanufacturing platforms.
We identified strongly with BPOG’s vision to accelerate the industry’s journey by identifying common biomanufacturer needs and sharing them openly with supply partners, academics, regional innovation hubs, regulators and government agencies. This will help to align directions and enable pre-competitive collaboration.
Ultimately, we wanted to help provide a structured approach to the assessment of the business impact of different technology options upon the various biomanufacturing scenarios and to help establish a consensus on the baseline reference process. And the best way to achieve this was to use our BioSolve Process modelling software.
We worked closely with BPOG to analyse a range of process configurations that relate to monoclonal antibody manufacture. The configurations represent the state of the industry today, including two different scales and modalities in terms of the upstream and downstream manufacturing options. The configurations selected represent a consensus of the participants in the roadmapping exercise.
The published roadmap provides an overview of the modelling work done and the conclusions made. It represents:
- A baseline of different process modalities.
- A consensus on the configuration and performance expectations as of today.
- A baseline whereby process improvements can be measured.
But we are also making the actual models available to BioSolve users, and non-users can access the key assumptions and outputs via our Manufacturing Technology Roadmap Tool and run their own scenarios in order to:
- Gain insight into cost drivers by looking in detail at the cost breakdown of different modalities (perfusion, fed batch, hybrid single use, stainless steel, continuous, batch).
- Compare the different modalities for themselves.
- Review the configuration parameters and evaluate potential improvements using, for the first time, a standard configuration that represents a consensus of the participating companies.
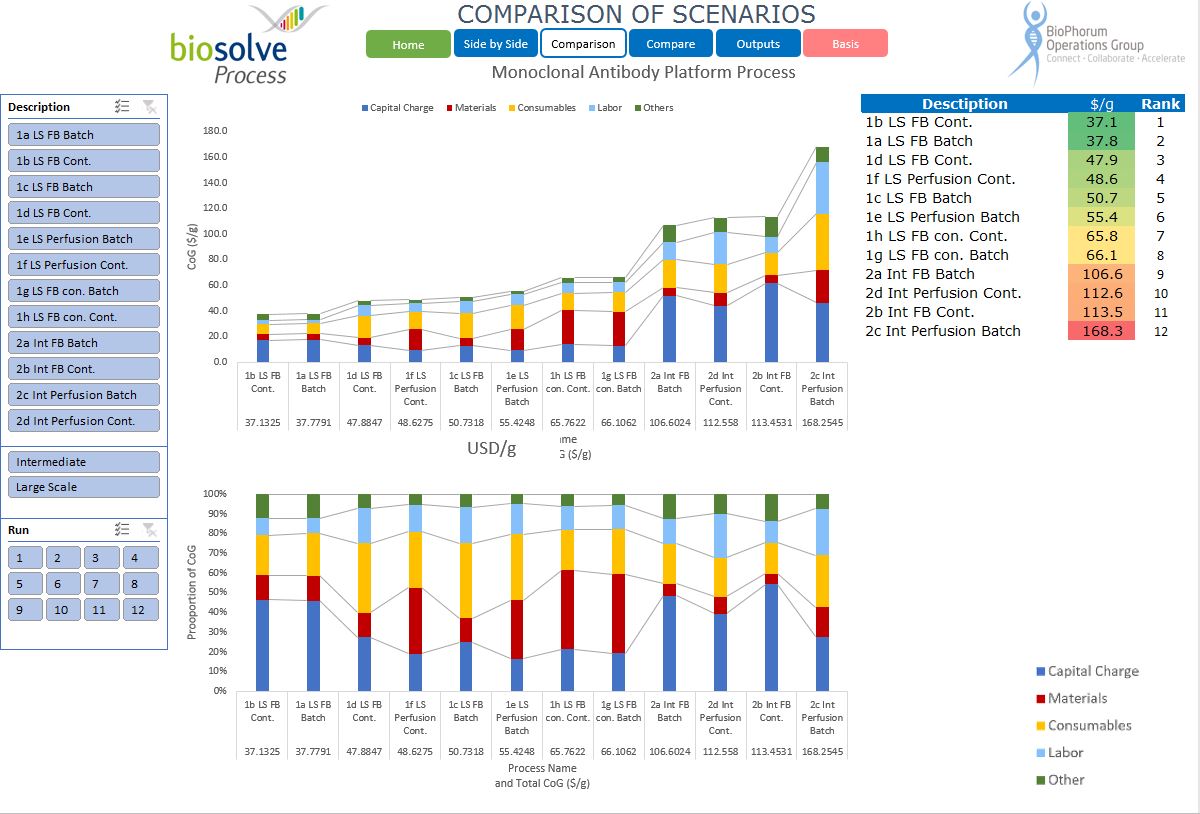
For the first time, this provides a foundation for comparing modalities and evaluating improvement from a commonly agreed baseline. Our tool allows those without BioSolve Process modelling software to examine the detail that lies behind the words in the report. This is a first significant step in setting goals for improvement and widening access to hard data. It is our intention widen the consensus and update the baseline models in a controlled way, and to use them to quantify and guide the various work streams looking at future improvements to manufacturing processes.
Andrew Sinclair, CEO, Biopharm Services



