Some biopharma companies have been running perfusion bioreactors in a continuous mode for several decades. The choice of whether to use continuous or not was dictated by the product, and little has been published on the economics of continuous perfusion processes.
In today’s blog we take a look the economics of perfusion and how it changes with scale. To accomplish this, we used the ability of BioSolve Process to readily model perfusion and fed batch processes at different scales, and to analyse different manufacturing configurations.
To understand the impact of perfusion costs in isolation, we defined three process configurations:
- Conventional (fed batch upstream, batch downstream)
- Hybrid (fed batch upstream, continuous downstream)
- Continuous (perfusion upstream, continuous downstream)
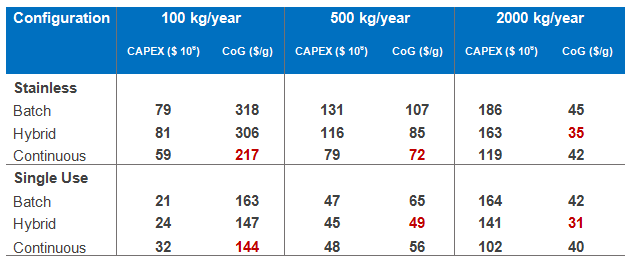
For the basis for this analysis we looked at a monoclonal antibody process at three scales (100kg/year, 500 kg/year and 2000kg/year) to represent the range we typically see in manufacturing. In the first case we looked at a typical high titre Mab at 4.5 g/L and the equivalent “traditional” perfusion process at 0.9 g/L.
Upstream modelling provides insight into the impact of titres and media usage/cost on the comparison of fed batch vs. perfusion. Two scenarios are presented: one based on a stainless steel facility and the second where based on a single-use technology.
The table of capital expenditure and operating costs (CoGs) provides interesting insights. First, we are able to distinguish the impact of perfusion on the process savings by looking at the savings associated with the hybrid process compared to the fully continuous process. The main difference between these two configurations is that the hybrid option uses batch bioreactors for the upstream operation whereas the continuous is based on perfusion.
Key observations:
- At lower throughputs, the economics favor the perfusion process.
- At a very large scale (2000 kg/year), the batch-based bioreactor is more economic.
- In this case, at about 500kg/year, there is a pivot point where we see the stainless process the perfusion bioreactor is favoured (although the savings are diminished), whereas for the single-use scenario the batch reactors are favoured
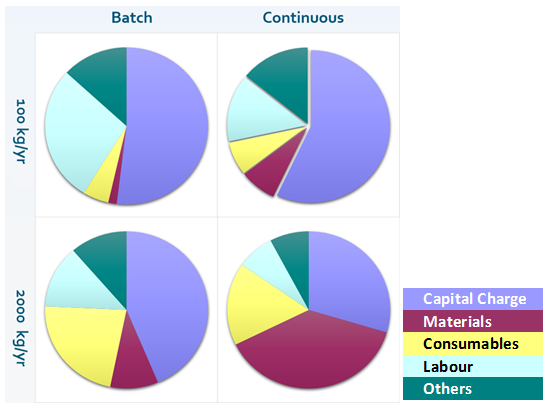
So what is happening? BioSolve provides further insight when we look at the cost of goods breakdown at the extremities of the capacity.
The material costs for the low-throughput process are not a significant contributor but, for a large-scale operation, the materials costs dominate for the perfusion-based process. Drilling into the numbers further, we find that the perfusion bioreactors in the continuous process use a large amount of media, used at a rate of two volumes of media per bioreactor volume per day (VVD), and we see that the cost of the media is a significant driver of CoGs at the larger scale.
This provides useful insight as it identifies targets for process improvement. We can conclude that perfusion bioreactors will be more attractive at lower throughputs, but there is potential for using perfusion in larger scale processing if we can:
- Reduce the cost of the media
- Reduce the media use efficiency by:
- Reducing VVD for a given titre
- Using a modern high cell density cell line producing higher product titres (this has to take account of increased costs associated with more complex media)
We feel this is an interesting topic and worthy of revisiting in the future. We would be interested in any feedback or thoughts on this subject.