What’s happening in downstream processing?
Welcome to the latest instalment of our continuous processing blog series. In Part 2 we considered the overall cost picture of the economics of continuous processing and touched upon the impact of perfusion versus fed batch – a theme we will pick up again later in the series. But today we will explore how the focus of continuous processing has moved downstream and how, in recent years, we have seen the development of operations that can be run in a continuous process.
First we need to consider what is meant by continuous operation.
From a process perspective, this involves moving from a discrete batch mode, where the batch size and frequency are used to define throughput, to a continuous processing environment, where there is a flow of material through a production line. The important concept here is that there is a continuous flow of material in and out of the unit operation.
What happens in the unit operation can be treated as a black box as long as there is a continuous flow in and out. Purists would argue that simulated moving bed chromatography is the only true continuous way of operation; however, all the continuous chromatography operations on offer are variants of using switched mode columns, with between two and more operated in sequence. In fact, any batch operation can be run in a continuous flow line and hence realize a lot of the benefits of continuous operation.
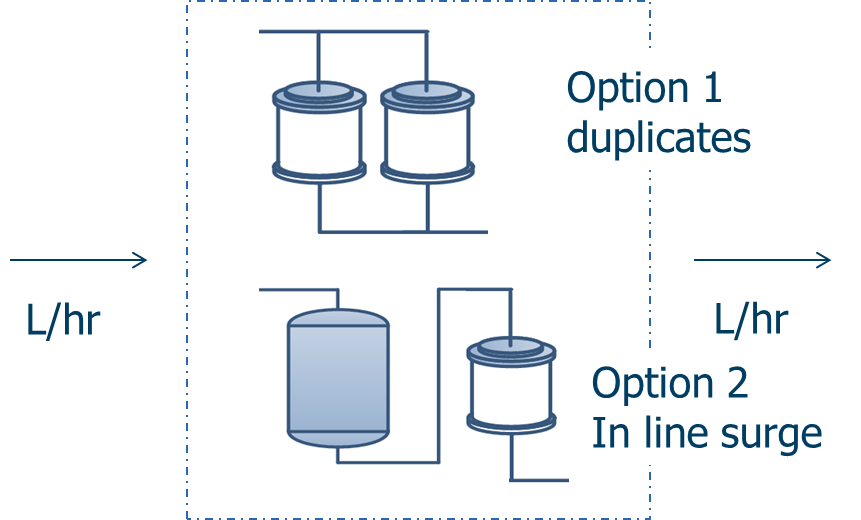
There are two basic options that are illustrated below using membrane absorbers.
In Option 1 the batch operation is duplicated. In this case the membrane absorber is run in parallel – one on standby and one in operation. The minimum size is based on the time taken to switch from standby to operation and the user can select any suitable size above the minimum.
In Option 2 a surge vessel is sized to accommodate the line flow during the switch from an exhausted membrane to a fresh unit. Surge vessels are often used to dampen flow fluctuations in the line. There is again flexibility in sizing the absorber.
The data
By applying these principles and employing technologies, such as switched mode multi column chromatography, which can be run in a continuous fashion, we can analyse the economics of continuous operation. The data presented below used BioSolve Process to compare conventional batch processing to continuous operation.
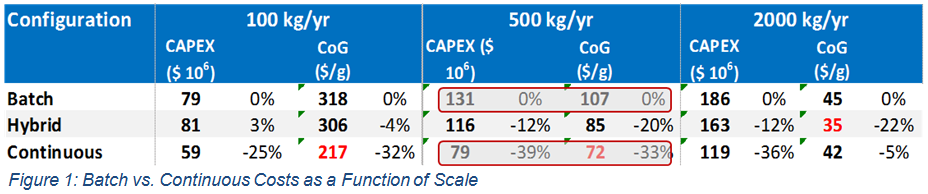
Consider first the downstream operation at the 500 kg/yr scale. In Figure 1, a significant reduction in both capital (39%) and operating costs (33%) is seen when moving from batch to continuous operation. Though interesting, it would be better understand how these savings are obtained.
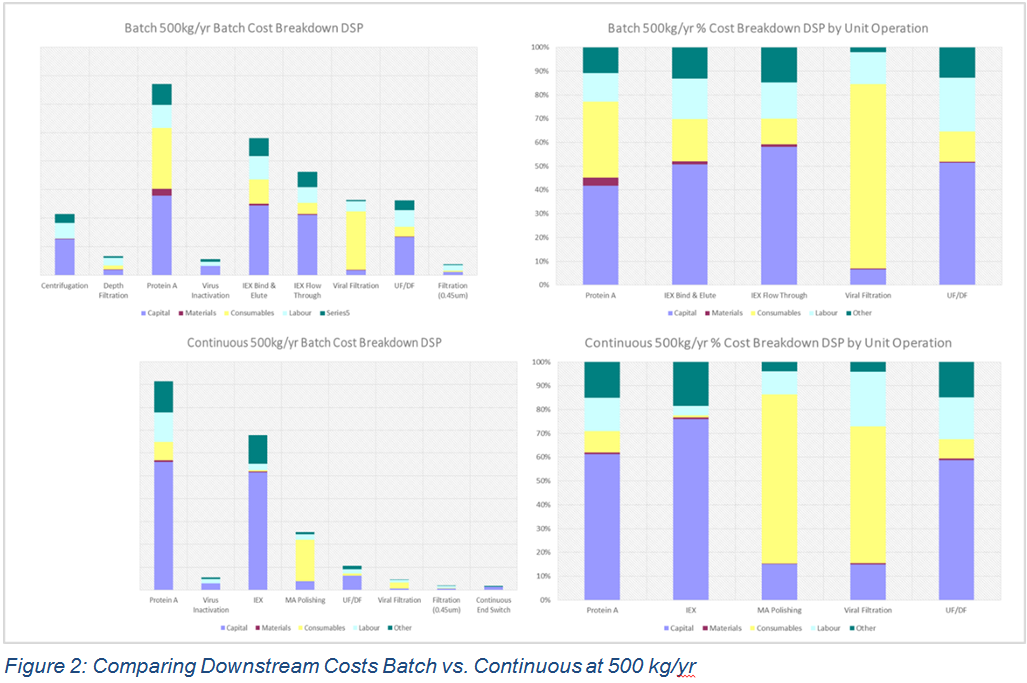
In BioSolve Process a batch cost breakdown can be generated by unit operation and cost category. This feature was used to generate Figure 2 – the left hand charts show the absolute costs and the right hand the normalised percentage breakdown. Upon inspection, the cost breakdown is very different:
- There are more unit operations contributing to overall cost of the batch process
- There is a more even distribution of costs in the batch process with the three chromatography steps dominating
- The continuous process costs are skewed to the two bind and elute chromatography steps
Comparing the Protein A column in the continuous process with the bind and elute Ion Exchange column in the batch process shows that the continuous column costs are dominated by capital costs associated with capital hardware, with less relative contribution from the resin costs. The implication is that capital hardware costs are relatively high and there could be significant scope for greater cost benefits if the hardware costs reduce as the market develops.
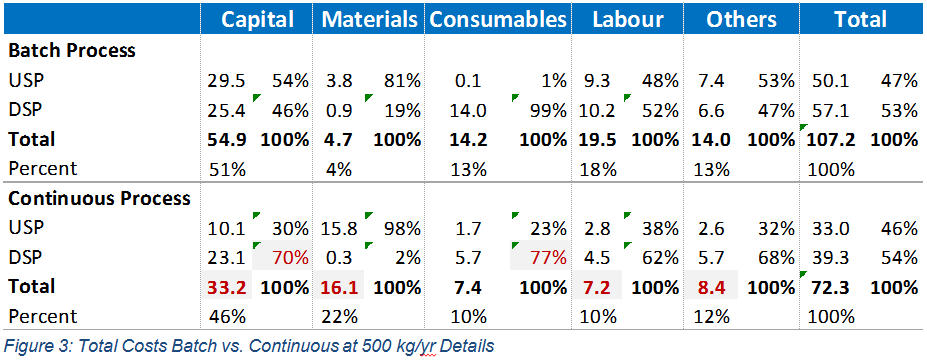
Finally, the total costs and the profile of cost distribution between upstream and downstream are considered in Figure 3. For capital costs, most of the continuous benefits are gained in the upstream area. Despite the much smaller scale in continuous downstream, there is little capital reduction. As expected for continuous operation, materials are dominated by upstream – more so than in the batch process. Also, for continuous processing there is significant consumable use upstream, which reflects the use of ultra-filter membranes in the cell separation device.
In conclusion, at the 500kg/yr scale some real benefit can be seen in moving to continuous processing, despite the much higher material costs associated with the perfusion bioreactor. It would appear the cost of chromatography hardware is high and that this is restricting the benefit we would expect in downstream processing when running continuously. We believe this reflects an immature equipment market in this area and we would expect costs to come down in the future, demonstrating future additional benefits for continuous processing.