The historical development of antibody drug conjugates is a well told yarn, where self-immolating reactions are scribbled on ESACT conference paper with PEGS free-issue pens. These multidisciplinary scientists and the molecules they make are writing a new storyline now.
Increased activity
With six approvals of new ADCs in the last 3 years, with expanded approved indications for the two well known, commercial ADCs – Kadcyla and Adcetris, and with 80 molecules in clinical trials, the field is buoyant. All of which has raised awareness to the global manufacturing requirements for commercial ADCs (1, 2, 3).
What scale of manufacturing might be required?
There are two approved HER2-targeting ADCs, Kadcyla and Enhertu; the sales projections of these out to 2026 are greater than Herceptin (4). With similar dosing strategies to Herceptin, the required quantities of these drug products will be 100’s kg. What’s more, Kadcyla is due to lose exclusivity in the next few years (5) and may see the development of biosimilar versions, potentially expanding the market demand.
So what scale of facility and investment is needed to produce blockbuster quantities of ADCs?
To help answer this, Biopharm Services has developed and tested a protein conjugation process model. The model represents an advanced starting point for developing a specific drug conjugate process model. There are so many different options of targeting molecule, conjugation chemistry, linker functions and drug molecules to utilise. Each variable has a different effect on the process capacity and operational costs. The starting model has utilised standard thiol-based reaction chemistry (reduction, partial oxidation, maleimide linker) followed by purification by UF/DF and chromatography. Options to include formulation, fill and lyophilisation are available within other standard BioSolve Process model options (requires customization support to adopt).
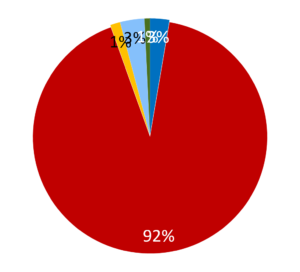
In order to produce 0.6 million 100mg vials (enough for 20,000 patients) in a 3 month campaign, a 200kg/yr capacity facility would need to conjugate 30 batches of 1.9 kg trastuzumab starting material. There are a few different options when it comes to analysing the required facility. If the biologic is already a manufactured product, as in the case of trastuzumab, then chilled/frozen biologic could be supplied to a dedicated conjugation facility. In this scenario, an estimated manufacturing footprint of 2000m2 would be required (not including office & ancillary). The capital equipment is calculated at $5.4M with a total capital setup expenditure estimated at $30M (including design, construction and validation).
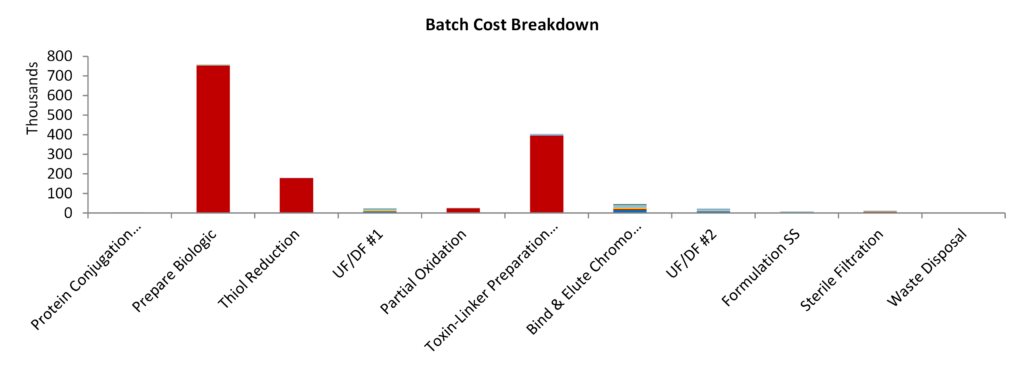
| Batches per Year | 120 | Capital | 23.4 USD Million | |
| Batches per Week | 2.59 | Cost of Goods | 777.5 USD/g | |
| Batch every | 2.7 | days | PMI | 16,819 |
| Batch Size | 1.9 | kg | Capacity | 228 kg/yr |
| DSP Recovery | 76% | Doses per Year | 2.3E+06 |
Illustration 1 – The production of 0.6 million doses/year of cysteine-linked antibody-drug conjugate drug substance in a dedicated ADC conjugation facility. The cost of monoclonal antibody biologic was treated as $300/g and the cost of drug-linker was $4000/g.
What other manufacturing options could be modelled?
The process model could be combined with the BioPhorum® standard mAb model (an industry standard mAb process model) to investigate a single facility to manufacture the biologic and conjugation as a part of the process. The production of pegylated biologics or chelator-conjugated antibodies for targeted radioimmunotherapy use a similar process flow, but the handling of high potency API’s at such large quantities for ADC manufacturing would require more specialised, controlled units with separate air handling, equipment and procedures. Illustration 2 shows a complete ADC drug substance process with biologics manufacture and conjugation in the one process flow.
Production of the drug-linker would always be in a separate chemical manufacturing facility and can be treated as raw material in the biologics/ADC facility.
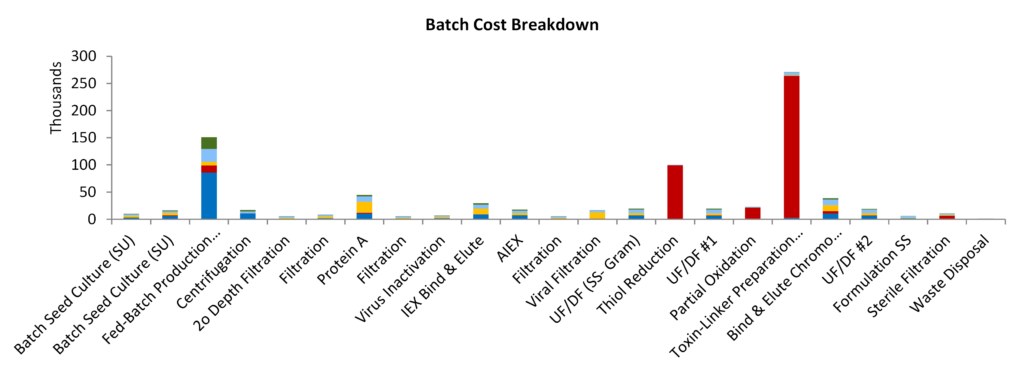
| Batches per Year | 128 | Capital | 110.69 | USD Million | |
| Batches per Week | 2.76 | Cost of Goods | 499.7 | USD/g | |
| Batch every | 2.5 | days | PMI | 25,552 | |
| Batch Size | 1.7 | kg | Capacity | 216.0 | kg/yr |
| DSP Recovery | 33% | Doses per Year | 2.2E+06 |
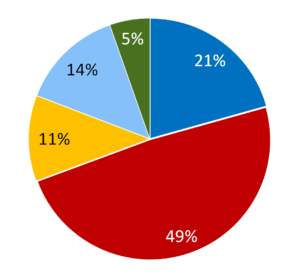
Illustration 2 – The production of 0.6 million doses/year of cysteine-linked antibody-drug conjugate – whole process contained in one facility. The cost of drug-linker was $4000/g
What are the key cost drivers?
The ongoing cost of goods of the ADC are dominated by the raw material costs in both manufacturing options. The drug-linker raw material cost alone is able to add between $200 to $2000/g onto the final ADC COGS, depending on the nature of the toxin. The cost of the biologic can also vary depending on whether it is outsourced (eg Illustration 1) or manufactured internally in a separate facility or combined facility (eg Illustration 2). This blog is not a full analysis or comparison between the different scenarios, but it does show how using an existing platform software can speed up the analyses. The data generated can guide the best use of existing capital and manufacturing infrastructure and help structure investment decisions. Or it could help understand the impact of outsourced costs, unit operation yields, scale of operation, QC costs, etc.
What about cost drivers at an earlier stage of development?
Given the critical cost of the toxin-linker, optimal conjugation efficiency will be an important consideration during development. We used the model in Illustration 1 to vary the molar ratios of toxin-linker to biologic. Increasing the ratio may be required to enable a higher drug-to-antibody ratio required for therapeutic effect or/and it may be required because of a decreased conjugation efficiency with some chemistries or procedures. The results are summarised in Illustration 3, for several different starting costs of toxin-linker (ranging from $4,000-$32,000/g). This type of data can help prioiritise work on process development.
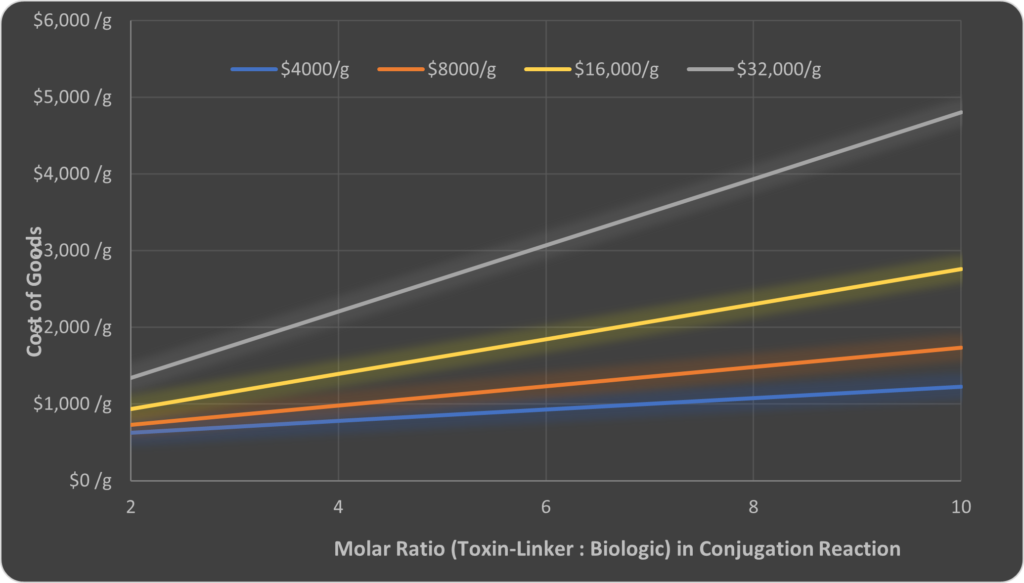
Illustration 3 – How the molar ratio of toxin-linker to biologic in the conjugation reaction effects the manufacturing cost of ADC drug substance (adapted from Illustration 1) for different input raw material costs of toxin-linker.
In summary
With the added complexity of the ADC supply chain comes an increase in the number of options available to secure drug supply at optimal costs. Recent approvals of ADCs point to an increased manufacturing demand for these more complex molecules. Manufacturing costs are considerably higher than monoclonal antibodies, up to 10 times higher, but there are opportunities to optimise the investment strategy for scale-up as well as reducing overall manufacturing costs. Models are easier to setup with existing platforms and we present the Protein Conjugation model as a starting point for your developments.
Please let us know if you are interested in the ADC/conjugation process model within BioSolve Process by contacting us at info@biopharmservices.com. If you are existing Licensee and trained user, you can download it from BioSolve Central now!
References
- ADC (Antibody Drug Conjugates) Contract Manufacturing Market, 2030 – ResearchAndMarkets.com
- Merck Announces € 59 Million Antibody-Drug Conjugate Manufacturing Expansion (Sept 2020). https://www.prnewswire.com/in/news-releases/merck-announces-eur-59-million-antibody-drug-conjugate-manufacturing-expansion-859792639.html
- Lonza Celebrates The Grand Opening Of A New Facility For ADC Payload Manufacturing (Nov 2020) https://www.outsourcedpharma.com/doc/lonza-celebrates-the-grand-opening-of-a-new-facility-for-adc-payload-manufacturing-0001
- Brown, A. Macrogenics gets to market, but what comes next? Evaluate.com https://www.evaluate.com/vantage/articles/news/snippets/macrogenics-gets-market-what-comes-next (2020).
- Busse, A. & Lüftner, D. What Does the Pipeline Promise about Upcoming Biosimilar Antibodies in Oncology? Breast Care (Basel) 14, 10–16 (2019).



